CREDIT: ARGONNE NATIONAL LABORATORY
Lithium, the lightest metal on the periodic table, plays a pivotal role in modern life. Its low weight and high energy density make it ideal for electric vehicles (EVs), cellphones, laptops, and military technologies where every ounce counts. As demand for lithium skyrockets, concerns about supply and reliability are growing.
To help meet surging demand and possible supply chain problems, scientists at the U.S. Department of Energy’s (DOE) Argonne National Laboratory have developed an innovative membrane technology that efficiently extracts lithium from water. Several team members also hold joint appointments with the Pritzker School of Molecular Engineering (PME) at the University of Chicago.
Right now, most of the world’s lithium comes from hard-rock mining and salt lakes in just a few countries, leaving supply chains vulnerable to disruption. Yet most of the Earth’s lithium is actually dissolved in seawater and underground salt water reserves. The problem? Extracting it from these unconventional sources has been prohibitively expensive, energy-hungry, and inefficient. Traditional methods struggle to separate lithium from other, more abundant elements like sodium and magnesium.
In salt water, lithium and other elements exist as cations – atoms that have lost one or more electrons, giving them a positive electric charge. The key to efficient lithium extraction lies in filtering out the other cations based on both size and degree of charge.
The new membrane offers a promising low-cost solution. It’s made from vermiculite, a naturally abundant clay that costs only about $350 per ton. The team developed a process to peel apart the clay into ultrathin layers – just a billionth of a meter thick – and then restack them to form a kind of filter. These layers are so thin they’re considered 2D. But, untreated, the clay layers quickly fall apart in water.
To solve this problem, researchers inserted microscopic aluminum oxide pillars between the layers, giving the structure the look of a high-rise parking lot under construction – with many solid pillars holding each floor in place. This architecture prevents collapse while neutralizing the membrane’s negative surface charge, a crucial step for subsequent modifications.
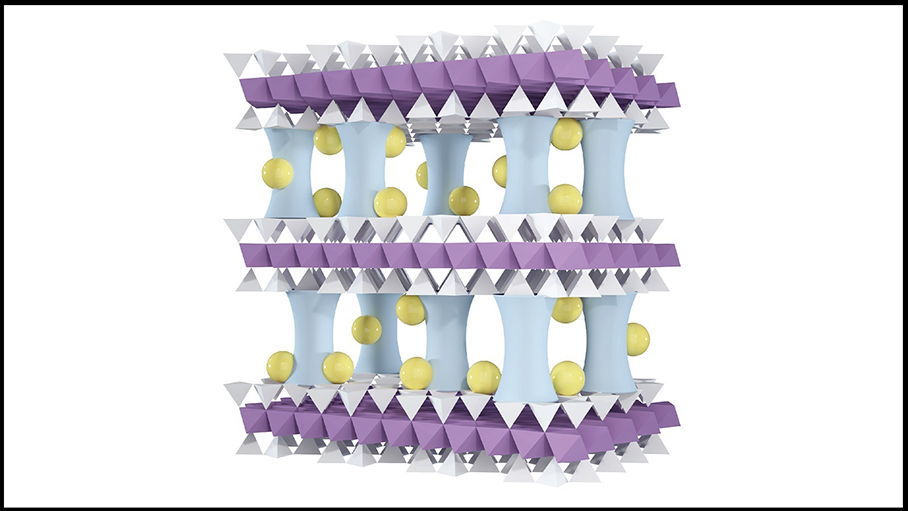
Next, sodium cations were introduced into the membrane, where they settled around the aluminum oxide pillars. This changed the membrane’s surface charge from neutral to positive. In water, both magnesium and lithium ions carry a positive charge, but magnesium ions carry a higher charge (+2) compared with lithium’s (+1). The membrane’s positively charged surface repels the higher charged magnesium ions more forcefully than it does the lithium ions. This difference allows the membrane to capture lithium ions more easily while keeping magnesium ions out.
The researchers believe this breakthrough could have broader applications, from recovering other key materials such as nickel, cobalt, and rare earth elements, to removing harmful contaminants from water supplies.
Latest from EV Design & Manufacturing
- Powering homes with EV batteries could cut emissions, save thousands of dollars
- Meviy introduces stainless steel passivation option for CNC, sheet metal parts
- December Lunch + Learn webinar with Fagor Automation
- December Lunch + Learn webinar with LANG Technik + Metalcraft Automation Group
- EVIO makes public debut with hybrid-electric aircraft
- Redesigned pilot step drill triples performance
- Green Energy Origin expands battery electrolyte manufacturing in North America, Europe
- What’s next for the design and manufacturing industry in 2026?